what is ionic bond
An ionic bond is the electrostatic attraction between oppositely charged ions. Ionic bonds are formed between two or more atoms by the transfer of one or more electrons between atoms.
 |
| Formation Of Ionic Bond And Factors Affecting It Variable Electrovalency |
Some examples are given as follows.
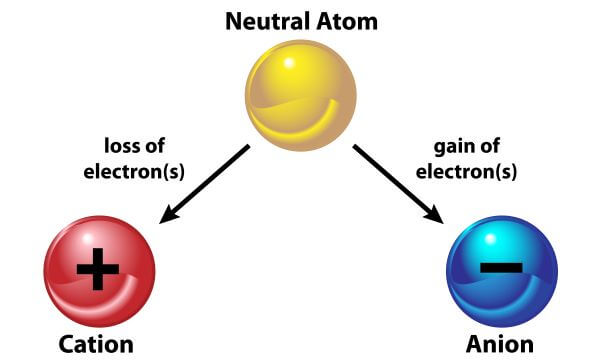
. By definition an Ionic bond is a type of chemical bond that occurs due to the permanent transfer of one or more electrons from one atom to another. The definition of ionic bond is a bond between atoms where electrons are mostly transferred from one atom to another. An ionic bond is a bond between two oppositively charged chemical species a cation and an anion. Ionic bonding is one of the most important types of chemical bonding.
Ions Ions are atoms that have gained or lost one or more electrons to. It is responsible for the very existence of the solid state which is a basis for all our physical and. The resulting compound is called an ionic. Ionic bonding When metals react with non-metals electrons are transferred from the metal atoms to the non-metal atoms forming ions.
Atoms that gain electrons make negatively charged ions called anions. It is one of the main types of bonding along with covalent bonding and metallic bonding. Ionic bonds are one of the two main types of chemical bonds. Ionic Bond Definition When two or more oppositely charged ions are held together due to the presence of electrostatic force the resulting bond is termed an ionic bond.
There is an ionic. Compounds having this type of bonding are called ionic compounds. Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions or between two atoms with sharply different electronegativities and is the primary interaction occurring in ionic compounds. An ionic bond is a chemical bond in which one or more electrons are wholly transferred from an atom of one element to the atom of the other and the elements are held.
Lets explore the process a little further. Ionic bonding refers to the complete transfer of valence electrons between atoms. The ionic bond between two atoms is the electrostatic force of attraction that holds together the ions of the combining atoms formed by the complete transfer of one. Charged chemical species form when neutral atoms or groups of atoms.
This exchange results in a more stable noble gas electronic configuration. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. An ionic bond is also termed an electrovalent bond. They form as a result of.
We say mostly because there is always some sharing of. Ions are atoms or groups of atoms with an electrostatic charge. Ionic bond refers to a type of chemical bond which generates two oppositely charged ions. An ionic bond is a chemical link between two atoms caused by the electrostatic force between oppositely-charged ions in an ionic compound.
Electron transfer produces negative ions called anions and positive ions called.
 |
| Ionic Bonding Wikipedia |
 |
| What Is An Ionic Bond With Two Suitable Examples Explain The Difference Between An Ionic And A Covalent Bond |
 |
| Definition Of Ionic Bonding Chemistry Dictionary |
 |
| Anlh39t2a4 Ffm |
 |
| Ionic Bonding Tec Science |
Posting Komentar untuk "what is ionic bond"